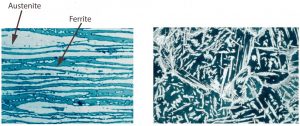
Heat input intake must be kept within a relatively narrow range when welding duplex steels, it needs have both an upper and lower limit. Upper limitations are due to the chemical composition of duplex steel, which is inclined to unfavourable precipitation formation when subjected to very high temperatures across a long period of time. It’s also important to note the embrittlement at 475 °C, as well as sigma phase formation at 600 °C. The risk of these phenomena grows as Cr content increases (this is also the reason for duplex steel’s max. 250 °C and super-duplex steel’s max. 220 °C limited working temperature). The impact of the welding temperature can diminish corrosion resistance and mechanical properties, especially if the interpass temperature is too high, or the heat can’t effectively dissipate from the weld due to the welded piece’s specific shape. For these reasons, the lowest welding heat intake possible is a fundamental requirement, though higher temperatures and lower cooling speeds may be favorable, so prescribing a heat intake minimum is also recommended.
Earlier, we mentioned that solidification is ferritic and that ferrite-austenite transformation happens in a solid state, so cooling speeds that are too high (with too little heat intake) limit austenite formation. For this reason, the time during which the weld pool cools from 1200 °C to 800 °C is crucial. Pre-warming is usually unnecessary, used at most in order to remove humidity from the steel’s surface (in this case, make sure that the reductive gas flame doesn’t increase the surface carbon content via diffusion processes). As the ferrite austenite transformation takes place between 1200-800 °C, the max. 250-300 °C preheating temperature can’t considerably decrease the speed of cooling from a higher temperature or thereby increase the amount of austenite that is formed. However, the cooling time period below 800 °C will increase (the cooling speed will decrease) and the risk of precipitation will grow, so preheating will probably have a negative effect. For this same reason, during multi-layer welding the interpass temperature must not exceed 150 °C.
When developing the technologies for welding duplex steels, the physical properties of the steel must also be taken into account. The heat conducting, heat expanding factors differ considerably from regularly weldable carbon steels, but also from classic austenitic corrosion resistant steel. As a result of all of this, we can expect above average deformation, and should pay special attention when putting together the structures (tackweld and welding order).
Duplex steels can be welded with the simplest and oldest shielded metal arc welding process (SMAW/MMA). The technique can be used flexibly with plate thicknesses that are at least 2mm thick. It’s recommended for onsite and repair welding, which is exceptionally useful for welding the pipes of “offshore” or chemical industry structures. In order to increase corrosion resistance, applying cleaning, pickling and passivation procedures is recommended. The acid used during the pickling process is more aggressive than those used with 304 and 316 types, and its activation time is longer. Of course, pickling time varies according to the material and the temperature of the environment. Covered electrode processes are often used combined with TIG/AVI welding, where the root pass is welded with a tungsten electrode technique, and the multi-pass welds are done with covered electrodes. To make thicker welds or when you’re welding equipment used in low temperatures, a basic coated electrode is recommended. In this case, the manufacturer can guarantee the industry standard level of power in as low as -60 °C environments.
Table 2. The chemical composition of welding materials used with various duplex steels
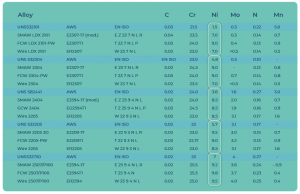
The composition of (TIG/GTAW, or AVI) and MIG/MAG (filler metal electrode with shielding gas) welding materials is basically the same. Table 2 [5] contains welding material types used with various duplex materials in each procedure. In addition to the composition of the welding material, the choice of shielding gas technology for the work gas and the gas protecting the root can influence the ratio of austenite-ferrite in the structure.
Though consumable electrode and shielding gas welding is impossible without welding materials, in certain circumstances TIG welding is possible without them if special protective gases are used. These circumstances may arise when welding the butt weld of thin plates. With this welding technique, the use of N2 containing shielding gases is an advantage. As the increased Ni-content in the welding material ensures a balanced ratio of ferrite-austenite, so too does the nitrogen from the shielding gas achieve this when no welding material is used. Nitrogen is a strong austenite forming element, and as it enters the base metal during the welding process it helps form austenite there.
Furthermore, nitrogen has the biggest impact on pittingcorrosion resistance and it also enhances mechanical properties in small doses. Nitrogen’s solubility at 1600 oC is 0,045% and it quickly grows as chrome content increases [6]. For example, austenite steel’s N solubility is around 0,4%. As mentioned before, too much N2 content in addition to the material’s N content can cause gas porosity from nitrogen enrichment during cooling and ferrite’s low nitrogen dissolving capacity. It should be noted, that tungsten electrodes “wear” faster due to the nitrogen content in shielding gases. The electrode needs to be ground and changed more often than when the welding is done in a pure argon atmosphere.
Pure argon is the commonly used shielding gas used when AVI welding duplex steels. With this gas, the majority of welding work can be done securely and cost-effectively. Argon-hydrogen mixes — which are often used to weld austenitic steels in order to increase the speed of welding — are not recommended, because cracks from hydrogen may form from the increased ferrite content of the weld. Argon-helium mixes offer the potential to increase heat intake, with a favorable impact on the viscosity of the material, and they broaden the setting options for the welding parameters. The weld arc and the heat input also increases together with the helium content. In summary, the AVI technique guarantees the “purest” good quality weld from the perspective of both corrosion resistance and mechanical properties, but it’s also inefficient. Its application in welding thin plates or preparing root welds is very advantageous.
The heat intake value needs to have an upper and lower limit when gas metal arc welding (MIG/GMAW) duplex steels. For example, if the welds are welded too thinly the heat intake will be too small, resulting in an undesired weld structure where too much ferrite has formed. In contrast, too much heat input will cause austenite formation, and heat input exceeding 2 kJ/mm increases the risk of the formation of intermetallic phases (sigma, chi), which considerably detracts from both mechanical properties and corrosion resistance. A maximum 1.5 kJ/mm heat input and max. 100-150 oC interpass temperature should be maintained when welding super-duplex steels [7]. In general, the best welding quality can be achieved with an industrial pulse mode welding machine. The correct welding parameters need to be individually defined and checked for each job.
Usually the same protective gases are used with gas metal arc welding as with austenitic steels. Pure argon is generally not used for gas metal arc welding, as the arc will be unstable and the penetration low. Usually argon rich gas mixed with oxygen or carbon dioxide is used. With argon-oxygen mixed gases (with about a 1-3% oxygen content) the arc is even, and the material transfer is spatter-free. Compared to argon and carbon dioxide mixes, the penetration shape is worse and the weld’s surface is more strongly oxidized. The penetration can be increased by increasing oxygen content, but this will further oxidize the welded joint, thus argon and carbon dioxide mixtures containing 2-3% CO2 are more broadly used as they ensure a deeper penetration and less oxidation [6]. Compared to regular austenitic steels the penetration is worse and the weld pool viscosity is low, which heightens the risk of weld defects.
If we add helium to the mix — usually 30% — the melt pool will be more fluid, which ensures a better surface between the weld and the base material. Compared to pure argon or argon carbon-dioxide mixtures the greater arc voltage means that in addition to the other parameters, the heat input is significantly greater. This is advantageous, especially when a balanced ferrite-austenite ratio is a strict requirement. In the event that the material’s nitrogen content is high, ferrite’s nitrogen solving capacity may trigger nitrogen enrichment during cooling, which increases the risk for porosity.
We can achieve good results when metal arc welding duplex steels with a consumable flux cored wire electrode (FCAW process). The productivity of welding with flux cored wire electrodes is greater than with solid wire, and the classic 15-25% carbon-dioxide argon mix or even an economical carbon-dioxide welding gas to shield. The liquid pool’s protection is guaranteed in part by the flux of wire, thus a higher active gas content is allowed. Other advantages of the process are a wide and even arc that lower the risk of weld defects, and a lower tendency towards porosity or spatter. Discoloration or oxidation on the surface of the weld is shallow, decreasing the need to clean the weld after welding. Disadvantages of the process are that the slag needs to be carefully removed. Any remaining slag will create an inclusion which will result in welding failure. With the right quality of wire and good parameters the slag will separate, leaving it easily removable from the weld’s surface.
This can also lead to joint flaws or perhaps slag inclusion formation. For these reasons, an above average (wider) root face, and more open beveling (bigger bevel angle) is recommended. Classic rutile flux cored wire can only be used in a PA- position. The preferred fine grained spray arc material transition can be effectively achieved with a regular welding machine. Using a welding machine on 4 rolling feeders and applying ceramic root protection achieves the best result. There are so-called fast cooling slag creating consumable cored wire electrodes for position welding. The quickly solidifying slag helps support the melt until it completely solidifies. The wire, suitable for position welding the slag, usually separates harder and the applicable amperage is also lower than that of classic flux cored wire. The impact value of the weld is lower by flux cored wire than those welded with a similar type of solid wire.
In order to ensure the corrosion resistance of duplex steels during the processes described above, using some root protection is recommended. One method is the aforementioned root protecting ceramic backing, the other is to use the right gas shielding on the root side to keep air away. Usually, the same gasses are used for root shielding as with austenitic steels [8]. Due to the risk of cold cracking formations, the gas shield’s hydrogen content should be avoided. For root protection, argon, nitrogen or their mixture can be used. Generally speaking, in order to avoid root-side oxidation and to increase corrosion resistance, the remaining oxygen content on the root-side should not exceed 30 ppm. As a rule, spot corrosion resistance grows as root-side oxygen content decreases. An oxygen content measuring tool is the best way to ensure this.
Last but not least, submerged arc welding (SAW/UP) can also be used to weld duplex steel. Due to the peculiarities of the method it can only be done in a horizontal position, but in addition to a high effectiveness, it can also be used exceptionally well with plates thicker than 10mm. Root passes are usually prepared with a different process. The melt through value is smaller than welding standard austenitic corrosion resistant steels. With the right flux, good mechanical properties can be achieved. For example, using a flux with a high basically, we can get the highest impact value even at low temperatures. The durability of welds prepared like this is great. The formation of a lot of welding pour and excessive mixing measures should be avoided. Heat input should be maximized at 3 kJ/mm-ben for 2205 and 2304 duplex types, 1 kJ/mm-ben for LDX2101 lean-duplex and 2507/P100 super duplex steel [7].
Sources:
[1] Yrjöla,P.: Stainless steel hollow sections handbook, Finish Constructional Steelwork Association (2008), Helsinki
[2] Herbsleb,G.: Werkst.u.Korros. (1982) 33, pp.334-40
[3] Truman,J.E: Proc.U.K.Corrosion-87, Brighton, Oct.26-28, pp.111-29
[4] Westin,E.M.,Olsson,C.-O.A.,Hertzman,S.: Weld oxide formation on lean duplex stainless steel, Corrosion Science 50 (2008), pp.2620-2634
[5] How to weld duplex stainless steels, Avesta Welding
[6] Young,H.P,Zin-Hyoung,L.: The effect of nitrogen and heat treatment on the microstructure and tensile properties of 25Cr-7Ni-1.5Mo-3W-xN duplex stainless steel castings, Materials Science and Engineering,A297 (2001), pp.78-84
[7] Böhler Welding Guide. (2010), Kapfenberg
[8] Linde hegesztési védőgázai, (2008), Linde Gáz Mo. Zrt, Répcelak