The properties of duplex steels
Duplex steels get their names for their special two-phase, approximately equal ferrite to austenite structure. Their favorable properties are due to this special microstructure, which combines the advantages of the two different types of corrosion resistant steels.
On the one hand, we have the ferrite and martensite “chrome steels,” whose high – over 18% – Cr content gives them relatively good toughness, while the structure ensures high strength and thus a good resistance to stress corrosion cracking in chloride containing environments. However, their resistance to spot corrosion is worse and their weldability is limited compared to austenitic corrosion resistant steels. In the event that the cooling speed during welding is high, these steels are in large part inclined to hardening, that is, they harden and become brittle due to the formation of martensite.
On the other hand, we have the austenitic corrosion resistant steels, which are easy to weld, ductile and have a high resistance to gap and crevice corrosion.
In duplex steels, the Cr content is between 20-26%, Ni content is between 3-8%, and these materials generally contain between 1.5-5-5% molybdenum. These alloys further increase spot corrosion resistance, which is clearly demonstrated in Diagram 1. The names, classifications and chemical compositions of the most commonly used duplex steels are listed in Table 1 [5].
Table 1. Chemical composition, properties and standardised symbols of various duplex steels

Weldability of Duplex Steels
Ideally, the micro-structure of duplex steels is close to 50% austenite and 50% ferrite (Diagram 2). This ideal microstructure can be achieved by annealing the steel for close to 5 minutes at 1020-1100 °C, and then cooling it (in water) in a “controlled condition.” Duplex steels appear in the middle of the “austenite + delta ferrite” area of the well-known Schaeffler diagram, which represents corrosion resistant material structures.
Diagram 2. The characteristic structure of duplex steel contains 50% ferrite in the raw material (left picture) which, after welding, is contained in the weld (right picture) [5].
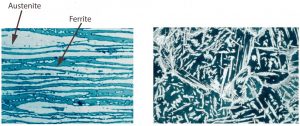
After welding, the ideal structure cannot be achieved after “unregulated” heating. Temperature differences and cooling speeds during welding alter the complex micro-structure, resulting in a modified structure that differs from the base metal and even throughout the binding’s different parts (Diagram 2). The impact of fast heat cycles in the heat-affected and melted zone may cause significant differentiation of the austenite-ferrite ratio from the base metal. Of course, this can impact the weld’s mechanical and corrosive properties as well. There is usually more ferrite in the heat-affected zone, while the amount of austenite is greater in the “middle” of the weld. After welding, generally 20-70% amount of ferrite form in various parts of the joint.
After fusion, the solidification is ferritic, and because the ferrite-austenite transformation happens in a solid state, an increased cooling speed can limit austenite formation. At the same time the high heat temperatures in the heat-affected zone may cause the unmelted austenite structure to become coarser from longer heating, or trigger a transformation to ferrite from slow cooling. Because of these two processes, the joint’s ferrite content can grow considerably, both in the heat-affected zone and in the melted part.
In order to achieve a proper microstructural balance in the weld, it’s crucial to choose welding material appropriate to the base metal. As a general rule, the welding materials used with duplex steels are basically the same as the base metal itself. However, the Ni content of welding materials is characteristically 2-4% larger than that of the base metal. Nickel’s austenite creating effect ensures a well-balanced austenite-ferrite ratio in the weld. Table 2 demonstrates the most commonly used welding materials and their compositions [5].
In some cases, especially when welding the root weld of steels with 22% Cr-content, higher chrome content containing welding materials are used in order to increase pitting corrosion resistance. But these welding materials, similar to their corresponding base metal, are more sensitive to the formation of intermetallic phases. As a result, ductility may decrease, so welding parameters must be thoughtfully chosen and strictly maintained. If the duplex or super-duplex welded joints must satisfy the highest requirements of corrosion resistance and mechanical properties, then overalloyed welding materials are recommended. In this case, an Ni-based alloy (where PRE=55…60) ensures the highest resistance to stress and pitting corrosion.




