Nickel, monel and nickel-molybdenum filler metals
Due to its protective oxide surface layer pure nickel is highly resistant to corrosion attacks even in highly chloridic environments. A typical base alloy is the Alloy 200 series, used for manufacturing chemical-, food- and power industry equipment. To weld the equipment, UTP 80 Ni and similar fillers have been developed (see Table 2.).
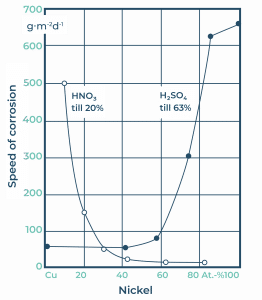
Pure nickel has a lower yield strength than general steels. Therefore pure nickel is not suitable for the manufacturing of structures working under relatively high stresses. Most alloying elements of nickel results in primarily solid solutions which has a strengthening effect. One of the alloying elements is copper, which can be completely dissolved in nickel. One example of this alloying system with a 30% copper addition to the nickel is MONEL. A representative example of this system is called the Alloy 400 series. Alloy 400 is typically used in so-called “offshore” applications, in systems working in sea water or similarly salt heavy environments. The welding fillers developed for this purpose, and their typical chemical compositions, are shown in Table 2. Nickel-copper alloyed welding demonstrates outstanding corrosion resistance, not only in high concentrated NaOH alkali medium but also in slightly acidic environments (see figure 2.).
Figure 2. Speed of corrosion in nickel-copper alloy.
The R405 alloy is part of the Alloy 400 series, and contains a small amount of sulphur (0.025-0.060%) in order to ease machinability. Welding of R405 is difficult. The dilution of base material should be kept as low as possible during welding, in order to avoid the segregation of sulphur. If Alloy 400 is to operate in a hydrofluoric or calcium fluoride environment, a stress relieving heat treatment between 550-650 C should be applied after repair welding to prevent stress corrosion cracking.
Different types of nickel-molybdenum alloys are similar to each other. Because of the molybdenum content, these materials have excellent corrosion resistance and especially high pitting corrosion resistance in chloridic, reductive environments. Previously, Alloy B types had to undergo a solid solution treatment at 1175 C after welding, due to the strong carbide precipitation in the heat affected zone, but the enhanced Alloy B-2 can be used in its welded condition. Because these alloys do not contain chromium, they have little resistant against oxidizing atmospheres. Alloy B-2 can be used in high temperatures in any concentration of hydrochloric acid. Depending on the welding process, either the UTP 703Kb basic electrode or UTP A 703 MIG wire or TIG rods are the most optimal.
Nickel-chromium-(cobalt-iron) alloyed fillers
Over the past 50 years, developments in the power plant industry, have resulted in built-in boilers, heat exchangers and turbines having to undergo three times more pressure and tension at up to 200-300 oC higher temperatures. This change led to the development of new materials and applications within nuclear power plants, conventional power plants and waste energy power plants as well. The main advantage of nickel-chromium alloys is their maintained strength at higher temperatures and consistent resistance against the effects of oxidation due to its automatically generated oxide surface layer. The most well-known such alloy type is Alloy 600, which contains at least 15% chromium. Furnace walls and reactors built from these kinds of materials can be welded with UTP 068 HH or similar fillers, which are excellent in both oxidizing or carburizing environments. The further developed Alloy 690 contains with 29% chromium content. This material is typically applied in joint welding or pipe cladding in nuclear power plants. Alloy 690 exhibits outstanding resistance against intergranular stress corrosion cracking. While the base material tends to crack from heat, it can be welded securely with the correct technologies, like the UTP 6229Mn or the Thermanit 690 type filler.
Nickel-chromium, nickel-iron-chromium and nickel-chromium-molybdenum alloys are susceptible to carbide precipitation, but this has no considerable effect on its corrosion resistance, so it does not require a heat treatment solution after welding. In order to decrease carbide precipitation susceptibility, low carbon and stabilizing elements containing filler metals are used. After welding Alloy 600, if the equipment is working in a high temperature, alkali environment, a stress-relief annealing is carried out at 900 oC in order to avoid stress corrosion cracking.
There are a lot of carbide forming companion elements in nickel alloys. The carbides form chemical compounds in nickel-based alloys. The presence and distribution of carbides have an effect on the mechanical properties of the material and heighten its resistance against creep at higher temperatures. Precipitation hardenable nickel alloys are used to manufacture devices working under high mechanical stresses. These alloys must be exposed to heat treatment after welding. Depending on the alloy type, the heat treatment temperature is between 600-760 oC. If high strength is not required than another filler type can also be used.
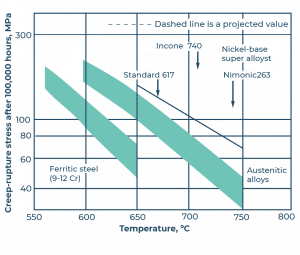
Alloy 617 is used as a base material for the pipes in conventional fossil-fueled boiler heat exchangers. As we can unequivocally establish from Figure 3, the creep limit of ferritic chromium steel is about 100 Mpa after 100,000 working hours at 600 oC, while Alloy 617 maintains a consistent creep strength, even at an elevated temperature of 700 oC.
Figure 3. Creep fracture of different types of materials as a function of temperature after 100,000 working hours.