First, the metallurgical properties of the two metals to be welded should be taken into account, as well as their differing chemical compositions which will determine the microstructure formed after the weld and thus the resulting joint’s physical properties. In addition to this, the welding parameters are also important, including heat input, the two metals’ physical and chemical qualities, and temperature. Finally, the newly joined components workability and other economic factors need to be considered.
There is no universal filler metal for every possible combination of welded nt heor operating condition in industry. In reality, choosing a good filler metal always requires a compromise. The fundamental requirement of the selected material is that the resulting weld and its heat affected zone not crack in operating conditions. The structure’s mechanical qualities should meet user requirement standards, and in order to guarantee its structural integrity, the weld’s strength should be equal to or greater than that of the weaker metal’s.
Selection depends largely nt he base materials and how the joint will be used. In addition to this, the characteristics of the weld also depend a lot on what process is used. This is especially true in the case of dissimilar metal welding, where care must be taken to keep weld dilution to a minimum.
The dilution value is defined by what percentage of the weld is made up of the melted material. This value is a function of the welding process and welding parameters. Table 1 depicts the average dilution of various welding processes.
The least amount of dilution is achieved with the least amount of heat input. The highest dilution levels are usually the result of Gas Tungsten arc welding (GTAW), Submerged arc welding (SAW), while the least amount of dilution is a result of specialized variations of Gas metal arc welding (GMAW).
If the dilution value is 20%, then each welded metal makes up 10% each of the weld’s chemical composition and the filler metal determines the remaining 80%. With certain processes, special properties also influence the dilution value. For example, processes that use shielding gasses are influenced by the gas composition.
When using the same parameters, shielding gases with higher energy conducting active gas content create a bigger melt through, which increases dilution. Gas mixtures that contain carbon-dioxide, oxygen or hydrogen are examples of these.
Naturally, shielding gases have additional side effects. For example, using austenite forming Nitrogen content in shielding gasses can be advantageous when welding corrosion resistant steels, however it can increase the risk of cracking.
In addition to those factors listed above, welding parameters also affect the dilution value. In general, combining lower welding power and/or tension and faster welding speed decreases heat input, which results in a smaller melt through and less dilution. When determining the process for welding dissimilar metals, the second factor to consider after the composition of the material is heat input, as the lowest heat input ensures the least amount of dilution.
In this article, we describe the filler metals and technological factors for frequently used dissimilar welds created with arc welding processes. The most frequent dissimilar metal weld is between unalloyed steel and some form of strongly alloyed corrosion resistant or heat resistant steel (ferrite, austenite, duplex, martensite or nickel based).
In spite of the fact that the two types of steel differ considerably in multiple physical qualities, including heat conductivity, heat expansion, magnetism, structure and corrosion resistance, this dissimilar metal weld remains a definitive weld in industrial structures.
These welds can be successfully welded using most arc welding processes. GMAW (FCAW), SMAW and GTAW are the most often used processes. If we weld carbon steel directly to corrosion resistant austenitic material, then a martensite structure with a low brittle-ductile capacity will form in the weld, resulting in cracking from thermal contraction.
Thus in practice, over-alloyed filler metal is usually used for dissimilar metal welds, which forms an austenite-ferrite structure after dilution and helps avoid cracking from thermal contraction.
The Schaeffler diagram depicts the typical structure of steels after rapid cooling and the risk of potential defects that may occur when they are implemented (including grain growth, sigma phase formation, cold and hot cracks) as a function of their composition. The effects of austenite forming elements are expressed with nickel equivalents, and the effects of ferrite forming elements are expressed with chrome equivalents.
In this way we’re able to predict the diluted structure of the weld as a function of the specified filler metal and dissimilar metals that are welded together. Diagram 1 presents a classic example of how the diagram should be used. In the diagram, the weld’s structure is indicated with a red dot, and is considered advantageous for both welding and implementing corrosion resistant steels.
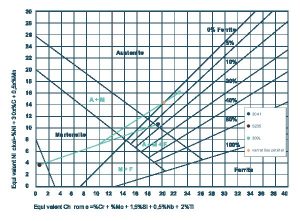
Diagram 1. The weld’s location on the Schaeffler diagram indicates a 20% material dilution when S235 unalloyed structural steel and 304L corrosion resistant steel is welded together using 309L filler metal.
At the same time, Diagram 1 also indicates that if the weld is prepared with an electrode made for carbon steel, the red dot would fall directly into the martensitic zone. In this case, the weld would most likely crack due to its brittle structure.




