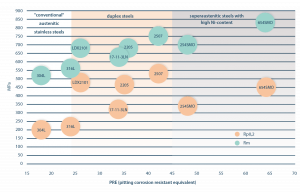
Duplex steels are favored for their good corrosion resistance and exceptional strength, as well as their relatively easy weldability. This is explored in detail in Diagram 1 [1], which illustrates the various duplex-, austenitic-, and super austenitic- steels’ mechanical properties and corrosion resistance. This article will dive into the weldability of duplex steels. We’ll introduce the effect of the material’s chemical composition, its corrosion resistant and mechanical properties, the metallurgical processes that take place during welding, as well as the different welding processes and the effects of their technological parameters.
Diagram 1. Corrosion resistance and solidity of various steels
PRE = % Cr + 3.3 % Mo + 30 % N
The equation demonstrates that Nitrogen content has the biggest impact on the PRE value, which isn’t surprising, as Nitrogen is a very strong austenite-forming element.
There are many published equations for calculating PRE similar to the one above, which seek to provide a more exact approximation for specific steel types. Of these, one of the more common ones is:
PRE=%Cr+3.3 (%Mo+0.5x%W)+a %N
(where “a”=16: for duplex and super-duplex steels, and “a”=30: for super-austenitic steels [3])
In this equation, the impact of tungsten’s pitting corrosion resistance is also defined. This is notable in the case of tungsten alloy super-duplex steels like 1.4501. In most stainless steels, the amount of nitrogen compared to the main alloys is minimal, thus the impact of multiplying factor “a” on pitting resistance is insignificant.
Pitting resistance is not measured using the usual weight loss based corrosion measurements. Though the signs of corrosion are clear, weight loss is minimal. Naturally there is a regulated measurement process to define pitting corrosion: The samples are “dissolved” in an aggressive chloride-ionic environment, and their surfaces are analysed after cleaning. These analyses are run in various temperatures, and the lowest temperature in which corrosion appears is labelled the CTP (critical pitting temperature) [4]. The analyses clearly prove that as corrosion resistance grows, the temperature at which corrosion begins rises linearly. Thus, duplex and super-austenitic materials can be used more securely in higher temperatures than traditional corrosion resistant steels.




