The behaviour of stainless steel bridge against corrosion
About 40% of bridges in the United States were built from steel, in Japan alone there are more than 50,000 steel railway bridges. Corrosion can lead to the progressive weakening of steel bridges’ structures, the reduction of their static capacity and increase of the dynamic vulnerability of the bridges [1]. Different types of steel are used for bridge construction, such as carbon steels, high strength steels and from the 1960s also weathering steels. The main damage is the appearance of rust, which can be eliminated not only by rust removal, but prevented altogether by building bridges from stainless steel.
Duplex stainless steels are increasingly used for bridges due to their good corrosion resistance and easy maintenance. Mameng at al [2] checked several bridges in their research to investigate their condition in the installation environment.
Two bridges over rivers and one footbridge near the seaside was examined. In one case corrosion patches (rust) appeared on the bottom of the bridges, but it was suspected that the beam had been rested on steel trestles at some point during their production, transportation or installation. In another case, pitting was observed also on the bottom of the bridge. This bridge spans a river near the sea. The pitting was caused by evaporation of salt water, where this steel surface was not exposed to washing by the rain. In both cases it was found that these surface defects do not affect the life of the bridge.
In the footbridge, discolouration was found along the welds, but these were not corrosion patches.

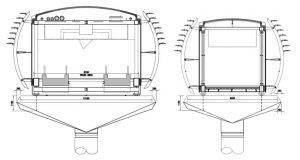
|

|
| Figure 1. Footbridge of Sölvesborg (Sweden) [3] | Figure 2. Galvanic corrosion on the footbridge [3] |
Galvanic corrosion can be found on cable fittings of the footbridge which was caused by the potential difference between the ‘love locks’ and the base material, but it did not affect the lifetime of the bridge. No special corrosion protection was applied to these bridges, this was left to the innate corrosion resistance of the steel. However, there are cases when the aim is to improve the protection of the material against corrosion; weather is by far not the only corrosive medium, for example acids, alkalis and drug substances used in the chemical, pharmaceutical or food industries can also be highly corrosive.
Many types of research investigate the corrosion resistance of nitrided steel to improve the protection of the surface which will be presented in Chapter 3.
References:
[1] Shuaicheng Guo, Ruizhe Si, Qingli Dai*, Zhanping You, Yunxiang Ma, Jiaqing Wang: A critical review of corrosion development and rust removal techniques on the structural/environmental performance of corroded steel bridges. Journal of Cleaner Production (2019) 233. 126-146
[2] S. H. Mameng, A. Backhouse, J. McCray, G. Gedge: Experience of duplex stainless steels as structural materials for bridges. IOP Conf. Series: Materials Science and Engineering 419 (2018) 012018.
[3] S. H. Mameng, A. Backhouse, G. Gedge: Experience of duplex stainless steels as structural materials for bridges: Results of seven inspections in European environment. EUROCORR 2019. Sevilla. 9-13. Sept. 2019.